 |
 |
- Search
| Arch Craniofac Surg > Volume 24(6); 2023 > Article |
|
Abstract
Background
Periosteum-mediated bone regeneration (PMBR) is a recognized method for mandibular reconstruction. Despite its unpredictable nature and the limited degree to which it is understood, it does not share the concerns of developmental changes to donor and recipient tissues that other treatment options do. The definitive role of the periosteum in bone regeneration in any mammal remains largely unexplored. The purpose of this study was to identify the genetic determinants of PMBR in mammals through a systematic review.
Methods
Our search methodology was designed in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines. We conducted a quality assessment of each publication, and evaluated the differences in gene expression between days 7 and 15.
Results
A total of four studies satisfied the inclusion criteria. The subjects and tissues examined in these studies were Wistar rat calvaria in two studies, mini-pigs in one study, and calves and mice in one study. Three out of the four studies achieved the necessary quality score of ≥ 3. Gene expression analysis showed increased activity of genes responsible for angiogenesis, cytokine activities, and immune-inflammatory responses on day 7. Additionally, genes related to skeletal development and signaling pathways were upregulated on day 15.
Reconstructing the mandible in young patients is considered uncommon and complex [1,2]. To date, approximately 30 cases of pediatric mandibular reconstruction have been documented in the English literature. Of these, 13 involved free flap reconstructions, 11 used non-vascularized reconstructions, and seven employed both techniques [1-30]. The intricacy of this procedure arises from the need for a thorough understanding of the developmental changes in bone and soft tissue at both the donor and recipient sites [26]. The choice of treatment for a mandibular defect, a crucial part of managing jaw tumors, varies based on the geographical and developmental context. Western centers often opt for primary or secondary reconstruction with vascularized osseous flaps and distraction osteogenesis. In contrast, developing healthcare systems like ours in Nigeria frequently use non-vascularized bone grafts, capitalizing on the high incidence of benign mandibular lesions in children [26-28,31]. A third treatment option, periosteum-mediated bone regeneration (PMBR) of the mandible, is commonly used in developing healthcare systems and is gradually gaining acceptance at Western centers [32,33]. To date, 32 case series involving 63 cases have been reported [32-63], with 44% (28/63) of these cases originating from Africa [33,34,41,50,52,54,55,57]. This trend may be due to limited facilities that hinder immediate or vascularized reconstruction. Despite the limited understanding and unpredictability of PMBR, there are no concerns about changes in donor and recipient tissues during growth and development.
In our previous clinical work on PMBR of the mandible in humans [33], we, like others, described it as an unexpected clinical phenomenon following periosteum-preserved mandibular resection by sub-periosteal dissection (Fig. 1A). This process is characterized by bone formation induced by the periosteum in an iatrogenic mandibular defect, provided three conditions are met: an intact periosteum, a mandibular segment as an inducer agent, and a young patient [32,39,64]. While these three conditions are generally required for PMBR, they are not absolute. There have been reports of the entire mandible regenerating [52] and adult periosteum undergoing bone regeneration, despite the preference to use PMBR with young periosteum [37,39]. This suggests that young periosteum may retain its osteocompetency despite increasing age [33]. PMBR is only indicated for benign lesions, as the periosteum needs to be preserved. Regarding its protocol, we reported that the regenerative potential of the mandibular periosteum begins as early as 7 to 10 days and can regenerate up to a bimandibular regenerative span of approximately 8 cm in length. We also noted that PMBR for the mandible can be classified as “complete” (i.e., regenerated bone occupying > 50% of the radiographic defect) or “incomplete” (i.e., irregular in outline and occupying < 50% of the radiographic defect) (Fig. 1B) [33].
While research on bone repair and regeneration is ongoing, the definitive role of the periosteum and the molecular pathways that regulate bone regeneration in mammals remain largely unknown [65]. Genetic determinants, as revealed through expression analysis, involve gene transcription and translation to functional gene products (RNA and protein) (Fig. 2). This is considered an accurate method for investigating genetic associations, as it provides insight into normal cellular processes [66]. Gene expression analysis can be broadly divided into RNA expression by transcription, protein expression by translation, and post-translational modification. However, RNA and protein expression levels are most commonly used for analysis. The techniques used to analyze gene expression include DNA arrays and real-time polymerase chain reaction (RT-PCR) for RNA expression, and Western blotting and gel electrophoresis for protein expression analyses [66,67]. The aim of this study was to conduct a systematic review of the genetic determinants of PMBR in mammals.
Our search methodology was designed in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines [68]. We used a Cochrane-style approach, employing MeSH terms and keywords related to periosteum, craniofacial and/or bone regeneration, as well as associated gene expression studies and techniques such as DNA array and RT-PCR. The initial search was conducted using the following tools: PubMed, Ovid Medline, and Web of Science.
Only English-language publications were reviewed, and the selected studies included cross-sectional studies, case-control studies, and controlled clinical trials. The titles and abstracts of these publications were reviewed to identify suitable articles, and the selected manuscripts were then proofread. Additional publications were retrieved and reviewed from the reference list of this initial search to identify potential papers that met the study’s criteria. All studies were required to use proprietary named kits for gene expression studies, in accordance with the manufacturer’s protocol. Articles that were purely clinical, related to pathology, conference proceedings, or only abstracts were excluded.
The quality of each publication was assessed using a modified version of a pre-existing tool, Strengthening the Reporting of Genetic Association studies (STREGA) [69]. This involved evaluating each article for the following elements: characterization of periosteum-mediated craniofacial bone regeneration, description of the case and control screening population, inclusion of gene or variant ID (e.g., National Center for Biotechnology Information rs identification, evaluation of gene function or Gene Ontology identifiers), and measurement of genetic associations. Additionally, the difference in gene expression between 7 and 15 days was reviewed. This time frame was chosen based on reports that bone regeneration can begin as early as 7 to 10 days [33].
All results were compiled into a data form for tabulation. We used the SPSS 27 software package (IBM Corp.) for statistical analysis. We recorded the sample size, age range, and mean or median of subjects with paired sample data during the expression analysis to provide descriptive statistical information. When a microarray was used to analyze differential gene expression, we applied a fold change threshold in accordance with the manufacturer’s protocol to determine the significance of gene expression.
The electronic search yielded 69 citations. However, 57 of these publications were discarded due to their lack of relevance to the search objectives. Specifically, 38 were descriptive studies, nine were conference proceedings, eight were observational studies, and two were duplicate studies. This left 12 publications for a full-text review. After a more detailed review, an additional eight studies were eliminated because they were purely clinical (observational) and had no correlation with gene expression. Consequently, only four studies [70-73] met the inclusion criteria (Fig. 3).
Three out of the four studies had a modified STREGA score of ≥ 3 (Table 1). The study subjects and tissues were Wistar rat calvaria in two studies, mini-pigs in one study, and calves and mice in one study. The techniques used to examine gene expression included microarrays, RT-PCR, and transcriptomics, with results assessed on days 7 and 15. In summary, the results indicated an increase in the activity of genes responsible for angiogenesis, cytokine activities, immune-inflammatory responses, and skeletal development. Genes associated with cytokine and immune-inflammatory responses, such as cytokines (interleukin-1β and interleukin-6), cathepsin K, and receptor activator of nuclear factor kappa-B ligand, were primarily implicated on day 7. In contrast, osteoblast-specific genes, including those coding for runt-related transcription factor 2, collagen type 1, osteonectin, and osteocalcin/osteopontin, were primarily implicated on day 15 (Table 2). Additionally, several biological signaling pathways, such as bone morphogenetic protein (BMP)-2, hedgehog, PDGF, Notch, and Wnt, were also implicated on day 15 (Table 3).
The bones in the craniofacial and appendicular regions develop differently. Specifically, the former originates from ectodermal neural crest cells of the closing neural tubes, which gives its periosteum and ossification patterns distinct characteristics compared to appendicular bones [74-76]. Craniofacial bone matures through an intramembranous process, characterized by various mesenchymal cells differentiating into bone-depositing osteoblasts or ossification centers, and eventually forming compact bone [77]. In contrast, appendicular bone develops through endochondral ossification, characterized by cell differentiation into chondrocyte-synthesized collagen, which matures through proliferation and then templates the shape of the future bone [78,79]. Despite these differences, intramembranous and endochondral bone developments share similar regulatory mechanisms [77,80].
Bone repair or regeneration is generally believed to be driven by the periosteum, but the precise role of the periosteum in bone regeneration remains unclear [77,78]. It consists of four successive phases: an initial inflammatory response and recruitment of osteo-progenitor cells, formation of a cartilaginous template, replacement of the template with immature (spongy) bone, and finally remodeling into mature bone. The periosteum is the primary driver throughout all four phases [80]. In the author’s opinion, an improved understanding of the periosteum’s role could provide a clear solution for bone repair or reconstruction, offering benefits such as low cost and reduced morbidity from a non-existent donor site. However, its unpredictability and lack of consensus among clinicians remain significant drawbacks. While few genetic association studies on PMBR have been conducted in humans, some have been carried out in animal models, which was the focus of this study.
The limited number of studies that met this study’s inclusion criteria highlights the scarcity of publications on this topic, even in animals. Additional searches revealed no reports on the underlying molecular mechanism of the periosteum in bone regeneration. One of the four studies that met the inclusion criteria was deemed to be below the required quality score due to a lack of Gene Ontology identifiers [73]. This same report also used RT-PCR, unlike the other three studies that used microarrays. The absence of Gene Ontology identifiers prevented specific gene expression interpretation, which would have facilitated the comparison of both techniques.
According to this review, skeleto-developmental and immune-inflammatory responses were the major events implicated in bone regeneration. The reports suggest that the inflammatoryimmune response was unique and different from that of typical soft tissue wound healing [81-83]. In addition to these two mechanisms, angiogenesis and neurogenesis also occurred. While new vessel formation is a well-recognized requirement for bone regeneration, neurogenesis was somewhat surprising. However, there have been reports suggesting that new nerve fiber formation promotes osteogenesis [84-86]. Furthermore, references to gene expression in PMBR were sought between 7 and 15 days because PMBR manifests clinically as early as 7 to 10 days. The upregulation of inflammatory and immune response at 7 days was expected, and this supports the initial inflammatory phase of bone regeneration. However, the additional responses of the I-kB kinase/NF-kB signaling pathway were interesting, as this is a known pathway reported to induce inflammation-induced bone loss [87]. In week 2, the upregulated pathways (transforming growth factor [TGF]-beta/BMP, Wnt, and Notch) are known to upregulate skeletal developmental gene (SDG) expression. While these pathways are well-known skeletal development pathways, BMP signaling is the only pathway suspected to be associated with bone regeneration, and BMP2 is suggested to regulate PMBR through periosteum-based target cells [88,89]. Additionally, the Wnt signaling pathway was another upregulated response noted in week 2. However, unlike the Bmp target cells, the Wnt target cells reside in fractured bones, suggesting that the Wnt pathway might influence more of bone repair than regeneration, albeit through a different mechanism from the Bmp signaling [90,91].
While PMBR shows significant potential for bone reconstruction, particularly in the mandible, the impact on developmental growth following pediatric mandibular reconstruction remains uncertain and requires further research. This uncertainty arises from several factors. For instance, the condyle is a site of mandibular growth, and whether it is involved or preserved could influence jaw growth after PMBR. While it is known that factors such as the patient’s age can affect the rate of growth, other factors such as radiotherapy and chemotherapy are not a concern because PMBR is not recommended for malignant lesions [12,26,33].
In conclusion, this review aimed to harmonize various reports on the intricate processes of PMBR in the mandible, focusing on gene expressions and signaling pathways. The findings suggest that the gene expression patterns of PMBR may be characterized by skeletal morphogenesis, which is regulated by SDGs and pathways. Immune-inflammatory genes seem to be predominantly upregulated in the first week, while SDGs and signaling pathways are upregulated in the second week. One limitation of this study is that it only characterized events based on whether gene regulation is up or down between the first and second week. Furthermore, due to the tissue heterogeneity in the periosteum, it was impossible to attribute a specific phenotypic or molecular event to a particular cell type. This was due to the lack of histological analysis, which meant the source of these events could not be localized. Additionally, the analysis only considered actively expressed genes at the transcription level, which may not correspond to the protein coded for or expressed. Therefore, integrating the assays used in these reports with immunohistochemistry or proteomics may provide a more accurate depiction of bone regeneration events, and could be a focus for future studies. Lastly, factors such as the patient’s age at the time of reconstruction and condylar preservation should be carefully considered during PMBR to ensure optimal results.
Fig. 1.
A case involving sub-periosteal dissection with arch bar placement. (A) A 10-year-old boy with right mandibular unicystic ameloblastoma (canine to ramus). (B) At 11 months postoperative, with complete periosteum-mediated bone regeneration.
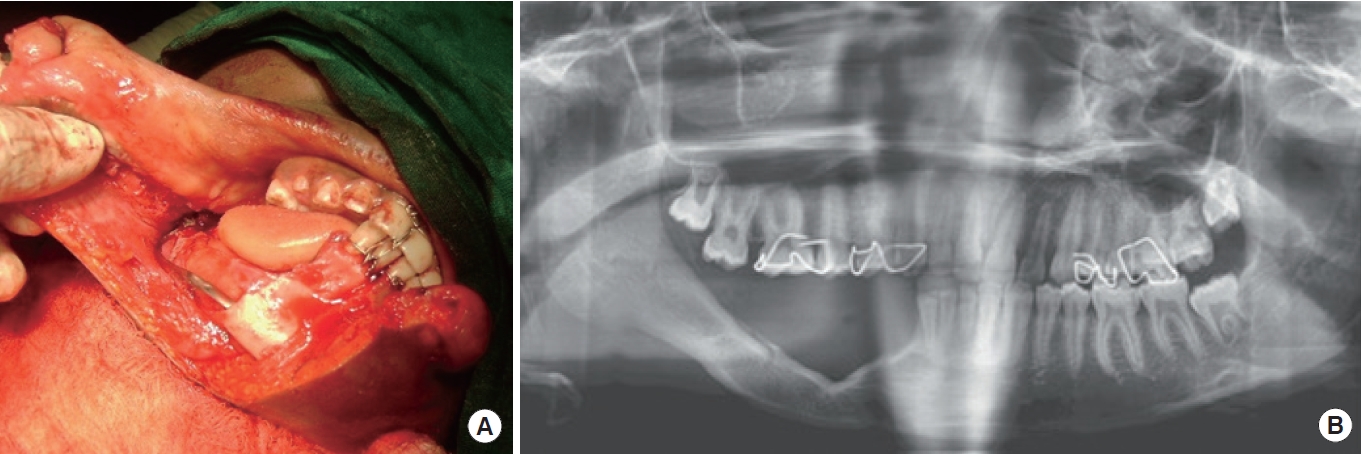
Table 1.
Modified STREGA quality score for each publication
| Publication | PMBR description | Use of controls | NCBI ID No./ Variant types | Validation of results | Reported data as risk ratio | Quality score (0–5) |
|---|---|---|---|---|---|---|
| Li et al. [70] | Yes | Yes | Yes | No | Yes | 4 |
| Al-Kattan et al. [71] | Yes | No | Yes | No | Yes | 3 |
| Ivanovski et al. [72] | Yes | No | Yes | No | Yes | 3 |
| Matsushima et al. [73] | Yes | Yes | No | No | No | 2 |
Table 2.
Publications with specimens and gene expression data on days 7 and 15
| Publication | Subjects | Mean age (mo) | Genes upregulated-day 7 | Genes upregulated-day 15 | Upregulated, No. (%) | Downregulated, No. (%) |
|---|---|---|---|---|---|---|
| Li et. al. [70] | 6 Mini-pigs | 18 | Inflammatory & immune response | SDGs, TGF-β /BMP, Wnt, and Notch pathways | 1,065 (55) | 877 (45) |
| Al-Kattan et al. [71] | 6 Wistar rats | 6 | Angiogenesis, NF-kappa β pathways, Inflammatory & immune response | SDGs, TGF-β/BMP, Wnt pathways | 361 (67) | 177 (33) |
| Ivanovski et al. [72] | 6 Wistar rats | 6 | Inflammatory and immune response | SDGs, TGF-β /BMP, and Wnt pathways | 41 (76) | 13 (24) |
| Matsushima et al. [73] | 3 Calves & mice | < 6 | ND | SDGs | 4 | ND |
Table 3.
Upregulated gene groups on day 15 (genes selected if the fold change between expression levels was ≥2.0)
| Publication | Screening assay | Upregulated SDGs with high fold change | Upregulated Wnt signaling pathway genes | TGF-β/BMP signaling pathway-related genes upregulated | Notch signaling pathway-related genes upregulated |
|---|---|---|---|---|---|
| Li et al. [70] | Microarray (APGA) | Th bs3, Dmp1, Pthlh, Osteocalcin, Msx1, Runx2, Collagen XI, XIII | Fzrb, Cpz, Wisp2, Wifi, Apc2 | Thbs3, Map3k1, Frzb, Tgfb3, Dlx5 | Notch4, Cfd, Foxc1 |
| Al-Kattan et al. [71] | Microarray (ARGA) | Bglap2, Dmp1, Bmp3, Col11a2 | Frzb, Dkk3 | ND | ND |
| Ivanovski et al. [72] | Microarray (ARGA) | Osteocalcin, Dmp1, Col13a1, Omd, Igfbp5, Mepe, Satb2, Runx2, Pthr1, Acan | Cpz, Wif1, Fzrb, Dkk3, Fzd6,8, Lrp4 | Thbs4, Bambi, Ltbp3, Pdgfrb, Bmp2 | ND |
| Matsushima et al. [73] | qRT-PCR | Collagen I & II, Runx, Bsp | ND | ND | ND |
REFERENCES
1. Smith A, Petersen D, Samant S, Ver Halen JP. Pediatric mandibular reconstruction following resection of oral squamous cell carcinoma: a case report. Am J Otolaryngol 2014;35:826-8.


2. Castellon L, Jerez D, Mayorga J, Gallego A, Fuenzalida C, Laissle G. Mandibular reconstruction for pediatric patients. J Craniofac Surg 2018;29:1421-5.


3. Posnick JC, Wells MD, Zuker RM. Use of the free fibular flap in the immediate reconstruction of pediatric mandibular tumors: report of cases. J Oral Maxillofac Surg 1993;51:189-96.


4. Hildago DA, Shenaq SM, Larson DL. Mandibular reconstruction in the pediatric patient. Head Neck 1996;18:359-65.


5. Lydaki E, Bolonaki I, Stiakaki E, Kambourakis A, Cordeiro PB, Meyers PA, et al. Immediate free flap mandibular reconstruction in osteosarcoma of the mandible in childhood. Pediatr Hematol Oncol 2000;17:335-40.


6. Eckardt A, Swennen G, Teltzrow T. Melanotic neuroectodermal tumor of infancy involving the mandible: 7-year follow-up after hemimandibulectomy and costochondral graft reconstruction. J Craniofac Surg 2001;12:349-54.


7. Nahabedian MY, Tufaro A, Manson PN. Improved mandible function after hemimandibulectomy, condylar head preservation, and vascularized fibular reconstruction. Ann Plast Surg 2001;46:506-10.


8. Phillips JH, Rechner B, Tompson BD. Mandibular growth following reconstruction using a free fibula graft in the pediatric facial skeleton. Plast Reconstr Surg 2005;116:419-24.


9. Fenton CC, Nish IA, Carmichael RP, Sandor GK. Metastatic mandibular retinoblastoma in a child reconstructed with soft tissue matrix expansion grafting: a preliminary report. J Oral Maxillofac Surg 2007;65:2329-35.


10. Warren SM, Borud LJ, Brecht LE, Longaker MT, Siebert JW. Microvascular reconstruction of the pediatric mandible. Plast Reconstr Surg 2007;119:649-61.


11. Bilkay U, Tiftikcioglu YO, Temiz G, Ozek C, Akin Y. Free-tissue transfers for reconstruction of oromandibular area in children. Microsurgery 2008;28:91-8.


12. Crosby MA, Martin JW, Robb GL, Chang DW. Pediatric mandibular reconstruction using a vascularized fibula flap. Head Neck 2008;30:311-9.


13. Li JS, Chen WL, Huang ZQ, Zhang DM. Pediatric mandibular reconstruction after benign tumor ablation using a vascularized fibular flap. J Craniofac Surg 2009;20:431-4.


14. Sinno H, Zadeh T. Desmoid tumors of the pediatric mandible: case report and review. Ann Plast Surg 2009;62:213-9.

15. Upton J, Guo L, Labow BI. Pediatric free-tissue transfer. Plast Reconstr Surg 2009;124(6 Suppl):e313-26.


16. Ducic Y, Young L. Improving aesthetic outcomes in pediatric free tissue oromandibular reconstruction. Arch Facial Plast Surg 2011;13:180-4.


17. Pierse J, Ying-Peng Wun E, Pellecchia R, Wollenberg J. Treatment of a rare ganglioneuroma with resection and reconstruction of the mandible: a case report and literature review. J Oral Maxillofac Surg 2014;72:748.

18. Zhang WB, Liang T, Peng X. Mandibular growth after paediatric mandibular reconstruction with the vascularized free fibula flap: a systematic review. Int J Oral Maxillofac Surg 2016;45:440-7.


19. Hu L, Yang X, Han J, Wang Y, Wang X, Zhu M, et al. Secondary mandibular reconstruction for paediatric patients with longterm mandibular continuity defects: a retrospective study of six cases. Int J Oral Maxillofac Surg 2017;46:447-52.


20. Malloy SM, Dronkers WJ, Firriolo JM, Nuzzi LC, Koudstaal MJ, Padwa BL, et al. Outcomes following microvascular mandibular reconstruction in pediatric patients and young adults. Plast Reconstr Surg Glob Open 2020;8:e3243.



21. Valentini V, Califano L, Cassoni A, Marco DM, Raponi I, Priore P, et al. Maxillo-mandibular reconstruction in pediatric patients: how to do it? J Craniofac Surg 2018;29:761-6.


22. Nam JW, Nam W, Cha IH, Kim HJ. Considerations for mandibular reconstruction in the pediatric patient following resection of malignant tumors. J Craniofac Surg 2019;30:e163-8.


23. Volk AS, Riad SSH, Kania KE, Davies L, Wirthlin JO, Pederson WC, et al. Quantifying free fibula flap growth after pediatric mandibular reconstruction. J Craniofac Surg 2020;31:e710-4.


24. Iconomou TG, Zuker RM, Phillips JH. Mandibular reconstruction in children using the vascularized fibula. J Reconstr Microsurg 1999;15:83-90.


25. Guo L, Ferraro NF, Padwa BL, Kaban LB, Upton J. Vascularized fibular graft for pediatric mandibular reconstruction. Plast Reconstr Surg 2008;121:2095-105.


26. Genden EM, Buchbinder D, Chaplin JM, Lueg E, Funk GF, Urken ML. Reconstruction of the pediatric maxilla and mandible. Arch Otolaryngol Head Neck Surg 2000;126:293-300.


27. Kolomvos N, Iatrou I, Theologie-Lygidakis N, Tzerbos F, Schoinohoriti O. Iliac crest morbidity following maxillofacial bone grafting in children: a clinical and radiographic prospective study. J Craniomaxillofac Surg 2010;38:293-302.


28. Rashid M, Tamimy MS, Sarwar SU, Rizvi ST. Benign paediatric mandibular tumours: experience in reconstruction using vascularised fibula. J Plast Reconstr Aesthet Surg 2012;65:e325-31.


29. Bianchi B, Ferri A, Ferrari S, Copelli C, Multinu A, Di Blasio C, et al. Microvascular reconstruction of mandibular defects in paediatric patients. J Craniomaxillofac Surg 2011;39:289-95.


30. Race, Ethnicity, and Genetics Working Group. The use of racial, ethnic, and ancestral categories in human genetics research. Am J Hum Genet 2005;77:519-32.



31. Sato M, Tanaka N, Sato T, Amagasa T. Oral and maxillofacial tumours in children: a review. Br J Oral Maxillofac Surg 1997;35:92-5.


32. Sharma P, Williams R, Monaghan A. Spontaneous mandibular regeneration: another option for mandibular reconstruction in children? Br J Oral Maxillofac Surg 2013;51:e63-6.


33. Okoturo E, Ogunbanjo OV, Arotiba GT. Spontaneous regeneration of the mandible: an institutional audit of regenerated bone and osteocompetent periosteum. J Oral Maxillofac Surg 2016;74:1660-7.


34. Adekeye EO. Rapid bone regeneration subsequent to subtotal mandibulectomy: report of an unusual case. Oral Surg Oral Med Oral Pathol 1977;44:521-6.

35. Ahmad O, Omami G. Self-regeneration of the mandible following hemimandibulectomy for ameloblastoma: a case report and review of literature. J Maxillofac Oral Surg 2015;14(Suppl 1):245-50.



36. Byars LT, Schatten WE. Subperiosteal segmental resection of the mandible. Plast Reconstr Surg Transplant Bull 1960;25:142-5.


37. Budal J. The surgical removal of large osteofibromas: the postoperative osteogenic capacity of the periosteum. Oral Surg Oral Med Oral Pathol 1970;30:303-8.

38. Cardinal L, Dominguez GC, Marodin AL, Rau LH. Unusual spontaneous mandibular regeneration of a large defect followed by orthodontics, alveolar distraction, and dental implant rehabilitation: a 10-year follow-up. J Oral Maxillofac Surg 2016;74:786-93.


39. de Villa GH, Chen CT, Chen YR. Spontaneous bone regeneration of the mandible in an elderly patient: a case report and review of the literature. Chang Gung Med J 2003;26:363-9.

40. Coen Pramono D. Spontaneous bone regeneration after mandible resection in a case of ameloblastoma: a case report. Ann Acad Med Singap 2004;33(4 Suppl):59-62.

41. Elbeshir EI. Spontaneous regeneration of the mandibular bone following hemimandibulectomy. Br J Oral Maxillofac Surg 1990;28:128-30.


42. Kamegai A, Mori M, Inoue S. Mandibular reconstruction using electrically stimulated periosteum. J Craniomaxillofac Surg 1990;18:8-13.


43. Espinosa SA, Villanueva J, Hampel H, Reyes D. Spontaneous regeneration after juvenile ossifying fibroma resection: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:e32-5.

44. Kazanjian VH. Spontaneous regeneration of bone following excision of section of the mandible. Am J Orthod Oral Surg 1946;32:242-8.


45. Keizer S, Tuinzing DB. Spontaneous regeneration of a unilaterally absent mandibular condyle. J Oral Maxillofac Surg 1985;43:130-2.


46. Kisner WH. Spontaneous posttraumatic mandibular regeneration. Plast Reconstr Surg 1980;66:442-7.


47. Martins WD, de Castro Avila LF. Partial spontaneous bone regeneration subsequent to mandibulectomy. J Contemp Dent Pract 2004;5:108-20.


48. Nagase M, Ueda K, Suzuki I, Nakajima T. Spontaneous regeneration of the condyle following hemimandibulectomy by disarticulation. J Oral Maxillofac Surg 1985;43:218-20.


49. Shuker S. Spontaneous regeneration of the mandible in a child: a sequel to partial avulsion as a result of a war injury. J Maxillofac Surg 1985;13:70-3.


50. Nwoku AL. Unusually rapid bone regeneration following mandibular resection. J Maxillofac Surg 1980;8:309-15.


51. Boyne PJ. The restoration of resected mandibles in children without the use of bone grafts. Head Neck Surg 1983;6:626-31.


52. Ogunlewe MO, Akinwande JA, Ladeinde AL, Adeyemo WL. Spontaneous regeneration of whole mandible after total mandibulectomy in a sickle cell patient. J Oral Maxillofac Surg 2006;64:981-4.


53. Khodayari A, Khojasteh A, Kiani M, Nayebi A, Mehrdad L, Vahdatinia M. Spontaneous regeneration of the mandible after hemimandibulectomy: report of a case. J Dent (Tehran) 2011;8:152-6.


54. Abdulai AE. Complete spontaneous bone regeneration following partial mandibulectomy. Ghana Med J 2012;46:174-7.


55. Adebayo ET, Fomete B, Ajike SO. Spontaneous bone regeneration following mandibular resection for odontogenic myxoma. Ann Afr Med 2012;11:182-5.


56. Throndson RR, Johnson JM. Spontaneous regeneration of bone after resection of central giant cell lesion: a case report. Tex Dent J 2013;130:1201-9.

57. Anyanechi CE, Saheeb BD, Bassey GO. Spontaneous bone regeneration after segmental mandibular resection: a retrospective study of 13 cases. Int J Oral Maxillofac Surg 2016;45:1268-72.


58. Ruggiero SL, Donoff RB. Bone regeneration after mandibular resection: report of two cases. J Oral Maxillofac Surg 1991;49:647-52.


59. Bataineh AB. Spontaneous bone regeneration of the mandible: in a case of osteosarcoma and literature review. Austin J Dent 2016;3:1031.
60. Guven O. Formation of condyle-like structure after treatment of temporomandibular joint ankylosis: literature review and long-term follow-up of two patients. Case Rep Med 2017;2017:9060174.


61. Whitmyer CC, Esposito SJ, Smith JD, Zins JE. Spontaneous regeneration of a resected mandible in a preadolescent: a clinical report. J Prosthet Dent 1996;75:356-9.


62. Rai S, Rattan V, Jolly SS, Sharma VK, Mubashir MM. Spontaneous regeneration of bone in segmental mandibular defect. J Maxillofac Oral Surg 2019;18:224-8.



63. Mesgarzadeh AH, Abadi AH, Keshani F. Seven-year follow-up of spontaneous bone regeneration following segmental mandibulectomy: alternative option for mandibular reconstruction. Dent Res J (Isfahan) 2019;16:435-40.



64. Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br 1975;57:36-45.


65. Ma JL, Pan JL, Tan BS, Cui FZ. Determination of critical size defect of minipig mandible. J Tissue Eng Regen Med 2009;3:615-22.


66. Ganguly P, Toghill B, Pathak S. Aging, bone marrow and nextgeneration sequencing (NGS): recent advances and future perspectives. Int J Mol Sci 2021;22:12225.



67. Buccitelli C, Selbach M. mRNAs, proteins and the emerging principles of gene expression control. Nat Rev Genet 2020;21:630-44.


68. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:n160.



69. Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, et al. STrengthening the REporting of Genetic Association Studies (STREGA): an extension of the STROBE statement. Genet Epidemiol 2009;33:581-98.


70. Li Z, Pan J, Ma J, Zhang Z, Bai Y. Microarray gene expression of periosteum in spontaneous bone regeneration of mandibular segmental defects. Sci Rep 2017;7:13535.



71. Al-Kattan R, Retzepi M, Calciolari E, Donos N. Microarray gene expression during early healing of GBR-treated calvarial critical size defects. Clin Oral Implants Res 2017;28:1248-57.


72. Ivanovski S, Hamlet S, Retzepi M, Wall I, Donos N. Transcriptional profiling of “guided bone regeneration” in a critical-size calvarial defect. Clin Oral Implants Res 2011;22:382-9.


73. Matsushima S, Isogai N, Jacquet R, Lowder E, Tokui T, Landis WJ. The nature and role of periosteum in bone and cartilage regeneration. Cells Tissues Organs 2011;194:320-5.



74. Runyan CM, Gabrick KS. Biology of bone formation, fracture healing, and distraction osteogenesis. J Craniofac Surg 2017;28:1380-9.


75. Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol 2004;275:1-11.


76. Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol 2012;366:34-54.


77. Stricker S, Mundlos S. FGF and ROR2 receptor tyrosine kinase signaling in human skeletal development. Curr Top Dev Biol 2011;97:179-206.


79. Nusspaumer G, Jaiswal S, Barbero A, Reinhardt R, Ishay Ronen D, Haumer A, et al. Ontogenic identification and analysis of mesenchymal stromal cell populations during mouse limb and long bone development. Stem Cell Reports 2017;9:1124-38.



80. Colnot C, Zhang X, Knothe Tate ML. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res 2012;30:1869-78.



81. Wang X, Yu YY, Lieu S, Yang F, Lang J, Lu C, et al. MMP9 regulates the cellular response to inflammation after skeletal injury. Bone 2013;52:111-9.



82. Kolar P, Gaber T, Perka C, Duda GN, Buttgereit F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin Orthop Relat Res 2011;469:3118-26.



83. Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev 2010;16:427-34.


84. Franquinho F, Liz MA, Nunes AF, Neto E, Lamghari M, Sousa MM. Neuropeptide Y and osteoblast differentiation: the balance between the neuro-osteogenic network and local control. FEBS J 2010;277:3664-74.


85. Portal-Nunez S, Lozano D, Esbrit P. Role of angiogenesis on bone formation. Histol Histopathol 2012;27:559-66.

86. Nunes AF, Liz MA, Franquinho F, Teixeira L, Sousa V, Chenu C, et al. Neuropeptide Y expression and function during osteoblast differentiation: insights from transthyretin knockout mice. FEBS J 2010;277:263-75.


87. Ruocco MG, Karin M. Control of osteoclast activity and bone loss by IKK subunits: new targets for therapy. Adv Exp Med Biol 2007;602:125-34.


88. Hashimoto K, Kaito T, Furuya M, Seno S, Okuzaki D, Kikuta J, et al. In vivo dynamic analysis of BMP-2-induced ectopic bone formation. Sci Rep 2020;10:4751.



89. Reyes R, Rodriguez JA, Orbe J, Arnau MR, Evora C, Delgado A. Combined sustained release of BMP2 and MMP10 accelerates bone formation and mineralization of calvaria critical size defect in mice. Drug Deliv 2018;25:750-6.












