|
 |
- Search
| Arch Craniofac Surg > Volume 24(6); 2023 > Article |
|
Abstract
The ultimate goal of cleft palate repair is to achieve an intact palate with the separation of the oral and nasal cavities. However, some patients develop an oronasal fistula in the secondary palate after palatoplasty. Postoperatively, a secondary palatal oronasal fistula may develop, leading to functional problems. In this study, we describe a patient with recurrent oronasal fistula and alveolar cleft with multiple failed previous reconstructions at another clinic. The oronasal fistula and alveolar cleft were repaired using a tongue flap and an iliac bone graft, respectively. The patient demonstrated excellent clinical progress with no recurrence of the oronasal fistula at the 1-year follow-up.
After primary palatoplasty, the incidence of fistulae recurrence ranges from 8.9% to 34%, and fistulae frequently require surgical correction [1]. Oronasal fistulae located on the hard palate are often difficult to repair using a local flap, even if they are small. Furthermore, oronasal fistulae of the anterior hard palate are more challenging to repair than other types of fistulae, and only a few local flaps are available. Therefore, large oronasal fistulae of the anterior hard palate are usually repaired using free or distant flaps. The tongue flap is an ideal surgical modality for repairing large, anteriorly-located oronasal fistulae because of its abundant blood supply and surgical flexibility. This report describes a surgical technique for repairing a recalcitrant oronasal fistulae using a flexible tongue flap.
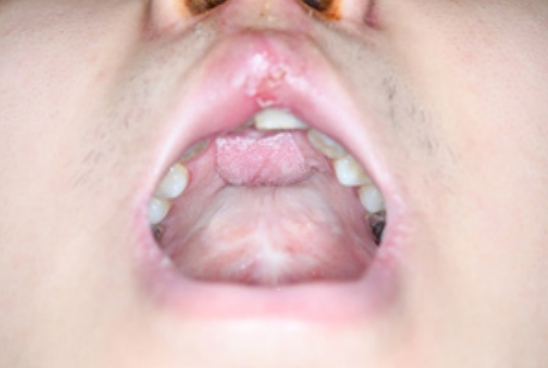
An 18-year-old man was referred to our outpatient clinic for the management of cleft lip and palate. He presented with an operated bilateral cleft lip and palate, a persistent oronasal fistula, and an alveolar cleft (Fig. 1). He had undergone four previous operations at another center, including closure of the palatal-oronasal communication and an alveolar cleft with an iliac bone graft. The oronasal fistula recurred at the anterior hard palate, which was closed at the patientŌĆÖs request due to fluid and food leakages into the nose. Additionally, the patient complained of poor pronunciation and nasal escape. According to the Pittsburgh classification, the patient had a type V oronasal fistula (Fig. 1A). The fistula involved the alveolar cleft and the anterior portion of the hard palate and was connected to the right nasal floor (Fig. 1C). To repair the defect and prevent recurrence, flaps should be sufficiently large enough to cover both the palatal and alveolar defects and allow for alveolar bone grafting. A tongue flap was chosen because it can provide a robust blood supply for bone graft healing and is sufficiently large to cover defects.
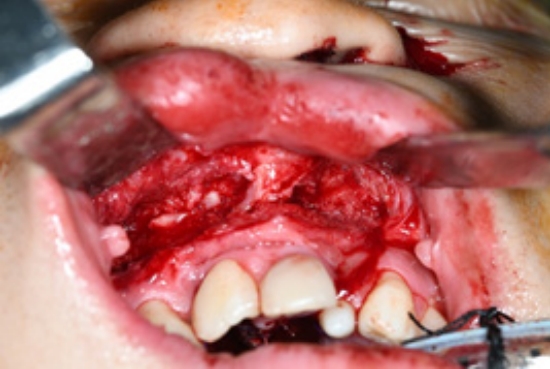
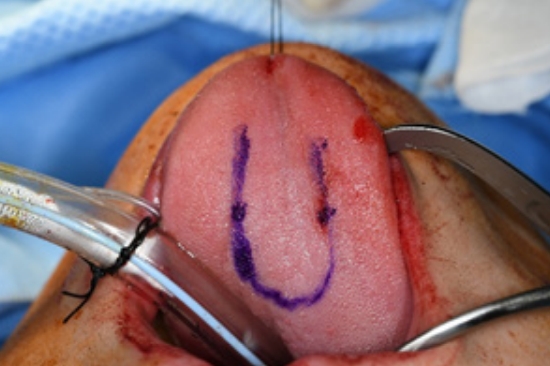
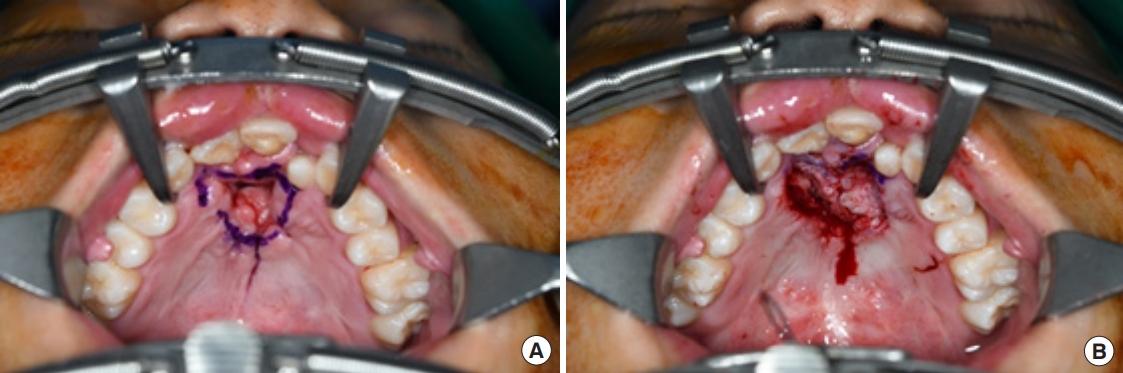
Surgery was performed under general anesthesia with oral intubation. A 1.5-cm-wide incision was made around the fistulous tract. The mucoperiosteal flap was elevated from the hard palate and turned over to repair the nasal side using 4/0 Vicryl (Fig. 2). Cancellous bone was harvested from the iliac bone and grafted onto the alveolar cleft. An additional incision was made along the gingivobuccal sulcus, and the anterior margin of the fistula was identified. The scar tissue surrounding the fistula was removed. The anterior margin of the fistula was approximated using gingival flaps prior to alveolar bone grafting (Fig. 3). Finally, a tongue flap was created to cover the oral defects. The base of the tongue flap was placed beneath the posterior border of the fistula with a width of 1.5 cm and a length of 3 cm, which was sufficient to cover the defect (Fig. 4). The flap was elevated to include a thin layer of the tongue muscle, with adequate bleeding control. The donor site was primarily closed with 4/0 Vicryl. After trimming the alveolar portion to fit the defect without tenting, the tongue flap was repaired with mattress sutures. Following surgery, the patient was permitted to follow a soft diet for 3 weeks. The pedicle was separated approximately 3 weeks after the first operation, and the tongue flap showed satisfactory viability without any wound problems. The oronasal fistula did not recur during the 1-year follow-up period, and the patient did not complain of further speech disability or donor-site morbidities (Fig. 5).
The primary goal of cleft palate treatment is to create an intact palate with separate oral and nasal cavities. However, some patients experience recurrent secondary palatal fistulae [1]. An oronasal fistula of the secondary palate may develop during the postoperative period in various locations, including the alveolus, hard palate, and junction between the hard and soft palate. Fistulae are common near the junction of the primary and secondary palates (type V) [2].
Various methods have been developed to close oronasal fistulae. Although simple local transpositional flaps can also be effective, they are only appropriate for small fistulae, and oronasal fistulae may still recur. Additional attempts to close the incision with local tissue often fail, as the thick and immobile scarred palatal mucoperiosteum leads to closure under tension, with subsequent flap necrosis and wound dehiscence [1]. Recurrent surgical interventions cause scar tissue to form around the fistula, making it difficult to be covered with only a local flap. Nonvascularized grafts, such as the dermis or cartilage, and tubed pedicle flaps from the abdomen, arm, neck, or cervicothoracic region, can be used as alternatives for fistula reconstruction.
Tongue flaps have been used to reconstruct defects of the lower lip, floor of the mouth, buccal mucosa, and palate. Their excellent vascularity and large amount of tissue availability in cleft palate surgery make them particularly suitable for the closure of extensive fistulae in palates scarred by previous surgeries [3-5]. A tongue flap has a well-vascularized pedicle with an excellent blood supply from the underlying genioglossus muscle. The tongue is supplied by the suprahyoid, dorsal lingual, sublingual, and deep lingual arteries. Owing to their vascularity, tongue flaps are particularly useful in large alveolar cleft repairs that require closure of oronasal fistulae. Tongue flap elevation is divided into two methods using either the anterior or posterior parts. Posterior flaps are suitable for treating defects in the soft palate, retromolar region, floor of the mouth, and posterior buccal mucosa [6,7]. Anterior flaps are useful in treating defects in the hard palate, anterior buccal mucosa, lips, and anterior floor of the mouth [8]. Our patient had a relatively large defect in the hard palate, recurrent oronasal fistula, and scarred tissues. Based on these characteristics, a tongue flap was considered the most suitable reconstruction tool for closing his complicated oronasal fistula.
One significant drawback of the tongue flap method is that it requires two surgical procedures. For the mucosal coverage of the defect, the tongue tissue is attached to the anterior maxilla for approximately 3 to 4 weeks. The pedicle is then separated from its base during a second operation that also includes flap division. In our case, the flap was divided 3 weeks after the first surgery. We confirmed the adequacy of blood supply to the distal part of the flap before the second operation, which showed stable viability after the surgery. Other reported complications of the tongue flap procedure include flap failure, partial flap necrosis, flap dehiscence, hemorrhage, and postoperative pain associated with temporary reduction in tongue mobility and sensitivity [9]. In this case, because the flap had an adequate length, width, thickness, and vascular supply, the patient did not experience any postoperative complications.
Kim et al. [5] reported a surgical protocol for repairing a bilateral cleft alveolus with a palatal fistula using a Y-shaped tongue flap, followed by secondary bone grafting. The difference in our case was that bone grafting was performed simultaneously with the tongue-flap procedure, which allowed successful closure of fistulae in the nasal, oral, and vestibular layers. Therefore, this procedure is recommended for cases in which the previous correction procedure has failed or is accompanied by palatal defects.
Reconstruction using buccal mucosal and facial arterial myomucosal flaps are common procedures for hard palate fistulae. However, the application of these methods is limited because covering a defect on the anterior hard palate is difficult. Therefore, if the defects are located on the anterior hard palate and are classified as Pittsburgh type V, a tongue flap can be a suitable choice for oronasal fistula reconstruction. The advantages of using tongue flaps include good stability, variable size and shape, low recurrence rates, and low donor-site morbidity.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Korea University Anam Hospital (IRB No. 2022AN0461).
Fig.┬Ā1.
Preoperative photographs and computerized tomographic images. (A) An oronasal fistula on the anterior hard palate. (B) The alveolar cleft connecting the oronasal fistula and alveolar tissue showing scarring due to previous surgery. (C) The fistula extending from the anterior hard palate to the right nasal floor and connecting to the alveolar cleft.
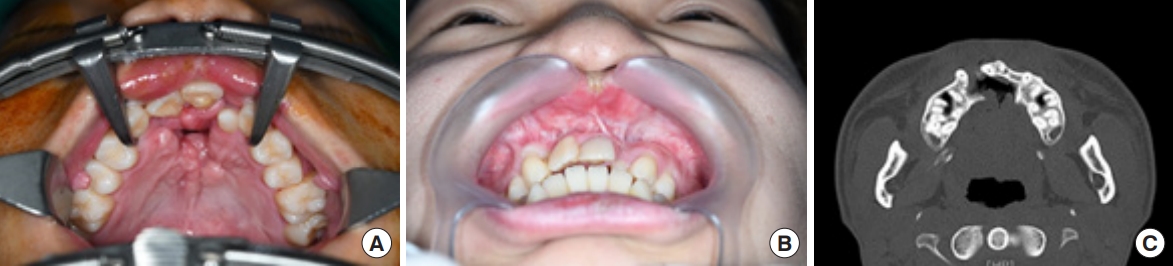
Fig.┬Ā2.
Preoperative design for the mucoperiosteal flap. (A) The mucoperiosteal flap is designed to mobilize the nasal layer. (B) The nasal layer is closed successfully through the flap.
REFERENCES
1. Posnick JC, Getz SB Jr. Surgical closure of end-stage palatal fistulas using anteriorly-based dorsal tongue flaps. J Oral Maxillofac Surg 1987;45:907-12.


2. Vasishta SM, Krishnan G, Rai YS, Desai A. The versatility of the tongue flap in the closure of palatal fistula. Craniomaxillofac Trauma Reconstr 2012;5:145-60.



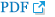
3. Gordon NC, Brown SL. Closure of oronasoantral defects: report of case. J Oral Surg 1980;38:600-5.

4. Smith TS, Schaberg SJ, Collins JT. Repair of a palatal defect using a dorsal pedicle tongue flap. J Oral Maxillofac Surg 1982;40:670-3.

5. Kim MJ, Lee JH, Choi JY, Kang N, Lee JH, Choi WJ. Two-stage reconstruction of bilateral alveolar cleft using Y-shaped anterior-based tongue flap and iliac bone graft. Cleft Palate Craniofac J 2001;38:432-7.


6. Vaughan ED, Brown AE. The versatility of the lateral tongue flap in the reconstruction of defects of the oral cavity. Br J Oral Surg 1983;21:1-10.


7. Johnson PA, Banks P, Brown AE. Use of the posteriorly based lateral tongue lap in the repair of palatal fistulae. Int J Oral Maxillofac Surg 1992;21:6-9.

- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 853 View
- 52 Download
- Related articles in ACFS
-
Scalp reconstruction using the reverse temporalis muscle flap: a case report2022 June;23(3)
Mandibular reconstruction using customized three-dimensional titanium implant2018 June;19(2)