|
 |
- Search
| Arch Craniofac Surg > Volume 24(5); 2023 > Article |
|
Abstract
Metastasis of lung cancer to the skin is uncommon, presenting in 0.22% to 12% of lung cancer patients, and it is extremely rare for skin metastasis to be the first clinical manifestation of lung cancer. In the few cases where skin metastasis has been reported as the first sign of lung cancer, the patients were typically heavy smokers or had preexisting respiratory diseases and symptoms. This prompted clinicians to consider skin metastasis of a pulmonary malignancy. Large cell neuroendocrine carcinoma (LCNEC) is a rare type of lung cancer that accounts for approximately 3% of lung cancers. LCNEC mainly metastasizes to visceral organs, such as the liver, bone, and brain, and it only shows metastasis to the skin in very rare cases. Herein, we report an unusual case of a metastatic skin lesion as the first sign of primary pulmonary LCNEC, in a 63-year-old woman with no pulmonary symptoms or personal history of smoking or pulmonary disease.
Large cell neuroendocrine carcinoma (LCNEC) is a rare subtype of lung cancer, accounting for 1% to 3% of all lung cancers, and is known for its aggressive behavior [1]. The aggressive nature of LCNEC, coupled with uncommon pulmonary symptoms such as cough, hemoptysis, and post-obstructive pneumonia, makes it difficult to diagnose in its early stages. Unlike other lung cancers, LCNEC is often discovered in advanced stages. Furthermore, approximately 40% of patients present with distant metastases to the liver, bone, and brain at the time of diagnosis [2,3]. A previous study reported that skin metastasis, an uncommon clinical symptom of pulmonary malignancy, is found in 0.22% to 12% of patients with lung cancer [4]. According to a report by Hidaka et al. [5], 15 out of 16 patients with skin metastasis of pulmonary malignancy were grade 4 and exhibited metastases to other organs such as the bone, brain, lung, and liver, in addition to the skin. The occurrence of skin metastasis as the initial presentation of lung cancer is extremely rare, and it suggests a poor prognosis. There have been few reported cases of skin metastasis from pulmonary malignancy as the first presentation [4,6,7]. However, it is rare for non-smokers to be diagnosed with pulmonary LCNEC through an initial skin presentation, without any respiratory or systemic symptoms; therefore, we report this case.
A 63-year-old woman presented at our hospital with an asymptomatic nodular mass on her right parietal scalp. The patient reported that the mass had developed two months prior to her visit and had gradually grown. The mass was round and firm, measuring 0.8Ă0.7 cm, with a hemispherical, raised protrusion of approximately 2 to 3 mm from the periphery. It was light pink in color, without any associated pain or tenderness, and exhibited ulceration. No other skin lesions were observed (Fig. 1). The patient had no specific medical history other than hysterectomy 10 years previously, no family history, and no systemic symptoms such as fatigue or weight loss. The patient requested removal of the mass due to discomfort. Based on its clinical features, we suspected the mass to be a benign skin tumor, such as a granuloma, dermatofibroma, or keratoacanthoma, and proceeded with an elliptical excision without further examination. A biopsy was subsequently performed. However, an initial pathological examination revealed intradermal-cord-like tumor invasion, with histological features similar to those of lung tumors with lymphovascular invasion (Fig. 2). Carcinoma was suspected, and we performed immunochemical tests, yielding the following results: CK7(+), EMA(+), CEA(+), HER2 (score 2), S-100(â), melan-A(â), CK20(â), estrogen receptor(â), progesterone receptor(â), TTF-1(â), napsin A(â), and CK5/6(â). Based on these findings, metastatic carcinoma was suspected. Positron emission tomography-computed tomography (PET-CT) and chest and abdominal CT were performed in consultation with the oncology department, and the PET-CT readings revealed a hypermetabolic lobulated nodule in the left lower lobe (LLL) and multiple hypermetabolic nodules in the lung, left pleura, peritoneum, liver, urethra, spine, pelvis, left tibia, and right fibula. Meanwhile, chest and abdominal CT showed a lobulated nodule in the LLL and multiple nodules in the left pleura, peritoneum, urethra, pancreas, pericardium, and liver (Fig. 3). A radiologistâs report suggested primary lung cancer in the LLL with multiple metastases, and consultation with a thoracic surgeon was requested for the lung biopsy.
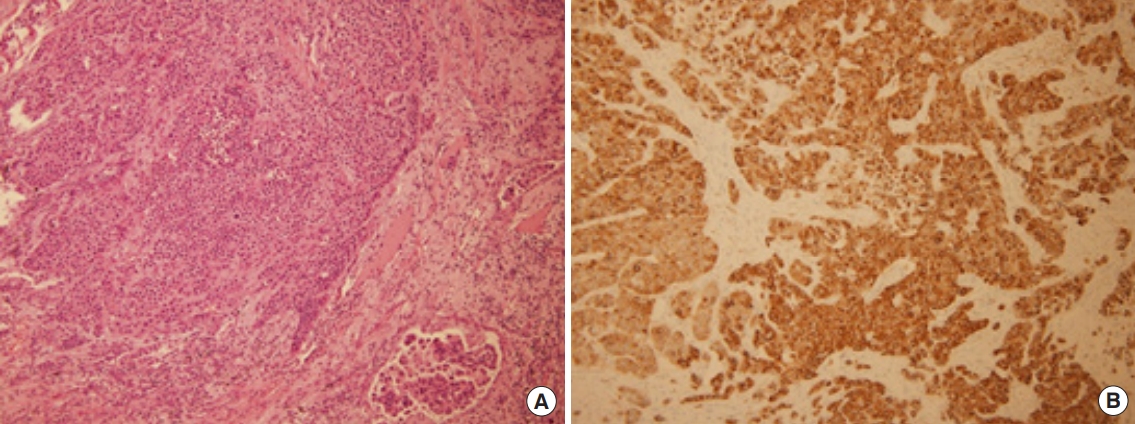
The diagnosis and treatment were carried out simultaneously via video-assisted thoracoscopic wedge resection, due to the high likelihood of malignancy and the presence of adhesion to the periphery of the LLL mass. The biopsy revealed monotonous tumor cells with lymphovascular invasion, and CD56 showed diffuse cytoplasmic positivity of the tumor cells (Fig. 4). TTF-1 was focally positive, the epidermal growth factor receptor score was 1+/60%, and chromogranin, synaptophysin, and CD56 were all positive, corresponding to LCNEC of the lung. The patient was administered three cycles of cisplatin and gemcitabine chemotherapy according to the guidelines, and radiotherapy (volumetric-modulated arc therapy, total: 44 Gy hip, 30 Gy left tibia and right fibula, 50 Gy urethra) was performed to manage cancerous pain due to bone metastasis.
LCNEC is a lung cancer subtype first proposed by Travis et al. in 1991 [1]. Unlike other pulmonary carcinomas with neuroendocrine morphology, such as atypical carcinoid and small cell lung carcinoma, which often present with comorbidities like carcinoid syndrome and paraneoplastic syndrome, LCNEC rarely has comorbidities and is primarily found in men who have been heavy smokers for several decades [1,2]. LCNEC typically follows an aggressive clinical course. Unlike other lung cancer subtypes, most pulmonary symptoms and clinical courses, such as cough, hemoptysis, and post-obstructive pneumonia, manifest in stage 4. When diagnosed at stage 4, the 5-year survival rate is 0%, and the survival period ranges from 4 to 12.6 months, indicating a very poor prognosis [2,3,8,9]. Skin metastasis occurs in approximately 0.22% to 12% of patients with distant metastases from pulmonary malignancy, with the lung being the second most frequent site after the breast for skin metastasis in visceral malignancies [4,10,11]. Despite some inconsistencies among studies, metastatic skin lesions are commonly observed on the scalp, trunk, head, and neck. These lesions can appear as solitary nodules or clusters, and they typically present as red or pink nodules firmly attached to the surrounding tissue. In some cases, they may be accompanied by inflammation or ulceration [10,11]. LCNEC mainly metastasizes to visceral organs such as the liver, bone, and brain, while skin metastasis is extremely rare [3,4]. Cases where the initial clinical signs of primary pulmonary malignancy appear in the skin or cutaneous layer are uncommon. To date, reports have documented solitary nodules [4] or cystic masses on the scalp [6], as well as hematomas on the forehead [12]. A case of LCNEC metastasizing to the scalp after the diagnosis of the primary cancer has been reported [13], but no case has been reported to date where skin metastasis was the initial presentation of primary malignancy. Notably, no cases have been reported in patients without a respiratory or smoking history, as seen in this case, for other lung cancer subtypes. Given that LCNEC primarily occurs in men (62.5% to 88%) and the proportion of smokers is very high (98%) [3,8,9], this case is considered extremely rare because the patient was a non-smoking woman without pulmonary or systemic symptoms. The treatment of LCNEC, particularly for advanced stages, includes chemotherapy as the primary treatment, surgical resection, radiotherapy, and targeted therapy. However, as a subtype that was only recently classified and has a low incidence, no treatment guidelines have been established [2,3]. The more advanced the stage, the poorer the treatment response. In this case, surgery, chemotherapy, and radiotherapy were performed immediately after the diagnosis of the primary cancer, but no significant effect was observed.
In conclusion, in cases where calp nodular masses are incidentally discovered, even if the patient is assessed to have a low risk of malignancy due to the absence of systemic or pulmonary symptoms or smoking history, surgical resection and biopsy should be performed as early as possible to differentiate malignant tumors.
Notes
Ethical approval
The report was approved by the Institutional Review Board of Eulji University Hospital (IRB No. EMC 2021-08-007-001).
Fig. 1.
Preoperative and intraoperative photographs. (A) A fibrous skin nodule on right parietal scalp of a 63-year-old woman. (B) The resected specimen, measuring approximately 0.8Ă0.7 cm.

Fig. 2.
Photomicrograph of the scalp specimen. The scalp specimen shows intradermal cord-like tumor invasion with histologic features similar to a lung tumor with lymphovascular invasion (hematoxylin and eosin, Ă100).
REFERENCES
1. Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB Jr, Nieman L, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma: an ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol 1991;15:529-53.

2. Fasano M, Della Corte CM, Papaccio F, Ciardiello F, Morgillo F. Pulmonary large-cell neuroendocrine carcinoma: from epidemiology to therapy. J Thorac Oncol 2015;10:1133-41.



3. Derks JL, Hendriks LE, Buikhuisen WA, Groen HJ, Thunnissen E, van Suylen RJ, et al. Clinical features of large cell neuroendocrine carcinoma: a population-based overview. Eur Respir J 2016;47:615-24.


4. Salemis NS, Veloudis G, Spiliopoulos K, Nakos G, Vrizidis N, Gourgiotis S. Scalp metastasis as the first sign of small-cell lung cancer: management and literature review. Int Surg 2014;99:325-9.



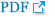
5. Hidaka T, Ishii Y, Kitamura S. Clinical features of skin metastasis from lung cancer. Intern Med 1996;35:459-62.


6. Kim HK, Kang SH, Kim WS, Kang SH, Kim WJ, Kim HS, et al. Scalp metastasis from an adenocarcinoma of the lung mimicking a cystic mass: case report and literature review. Arch Craniofac Surg 2022;23:237-40.



7. Gupta V, Bhutani N, Marwah N, Sen R. Scalp metastasis as an initial presentation of lung adenocarcinoma: a case report and literature review. Int J Surg Case Rep 2017;41:327-31.



8. Takei H, Asamura H, Maeshima A, Suzuki K, Kondo H, Niki T, et al. Large cell neuroendocrine carcinoma of the lung: a clinicopathologic study of eighty-seven cases. J Thorac Cardiovasc Surg 2002;124:285-92.


9. Hiroshima K, Mino-Kenudson M. Update on large cell neuroendocrine carcinoma. Transl Lung Cancer Res 2017;6:530-9.



10. Alcaraz I, Cerroni L, Rutten A, Kutzner H, Requena L. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol 2012;34:347-93.

11. Lee JH, Ahn SJ, Kim HJ, Jang SE, Noh GY, Kim HR, et al. Cutaneous metastasis from lung cancer: a single-institution retrospective analysis. Tuberc Respir Dis 2011;70:139-42.

- TOOLS